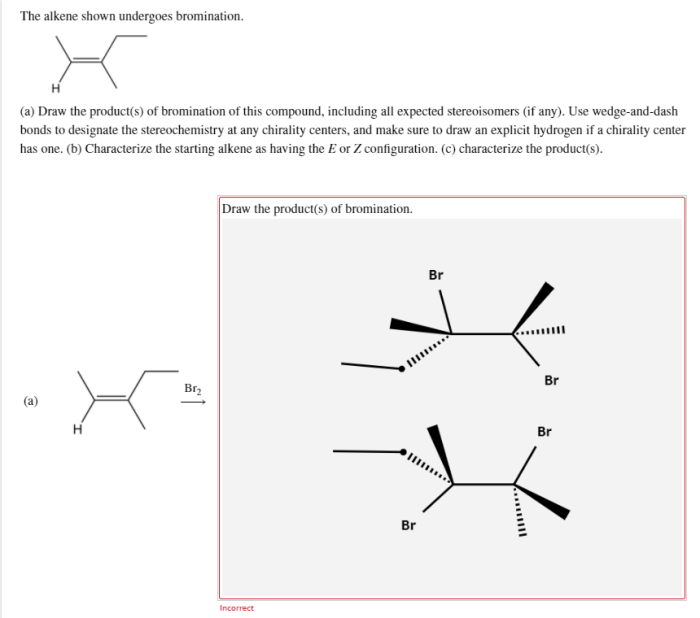
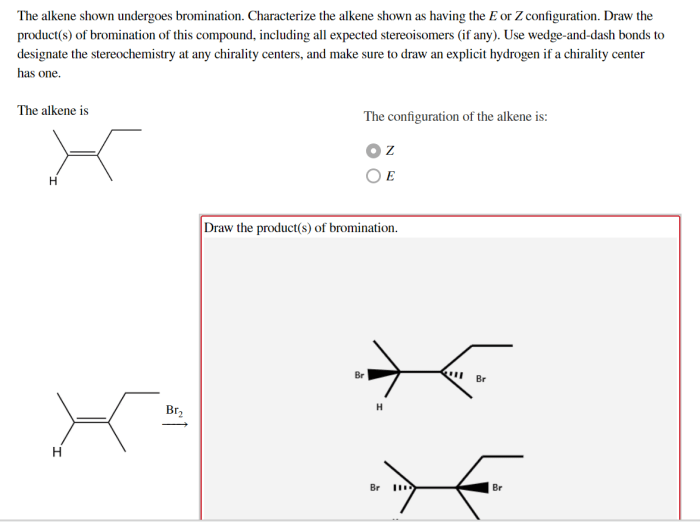
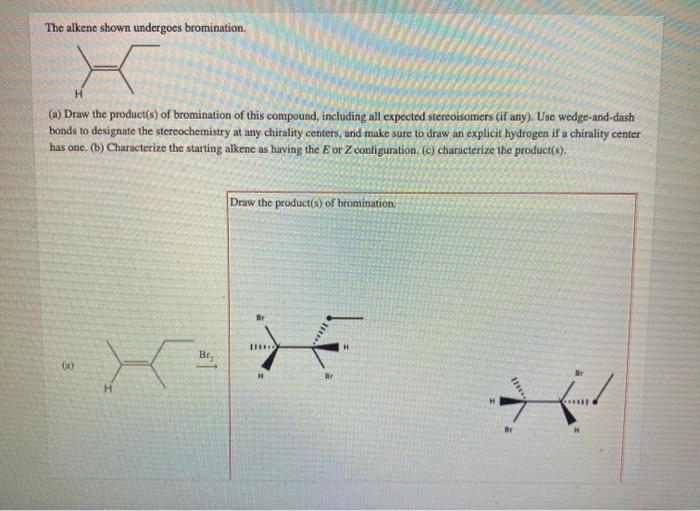
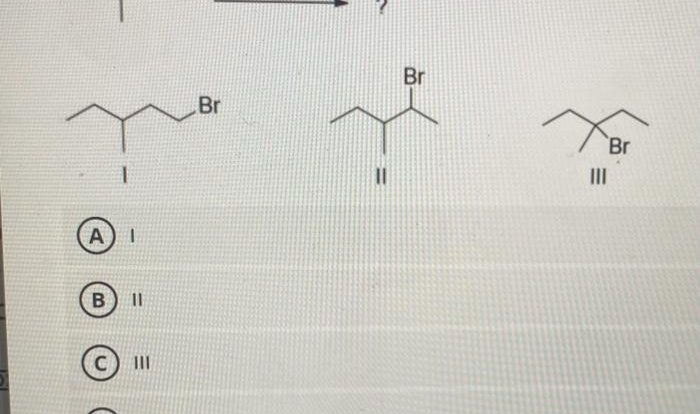
The alkene shown undergoes bromination, a captivating chemical reaction that unveils the intricacies of organic synthesis. This process, characterized by the addition of bromine to an alkene, holds immense significance in the realm of chemistry, providing a versatile tool for the construction of complex molecules and unlocking a plethora of applications.
Delving into the mechanism of alkene bromination, we uncover a fascinating interplay of electrophilic addition and radical intermediates. The regioselectivity and stereoselectivity of this reaction, governed by a delicate balance of electronic and steric effects, offer a glimpse into the intricacies of molecular behavior.
Alkene Bromination Reaction: The Alkene Shown Undergoes Bromination
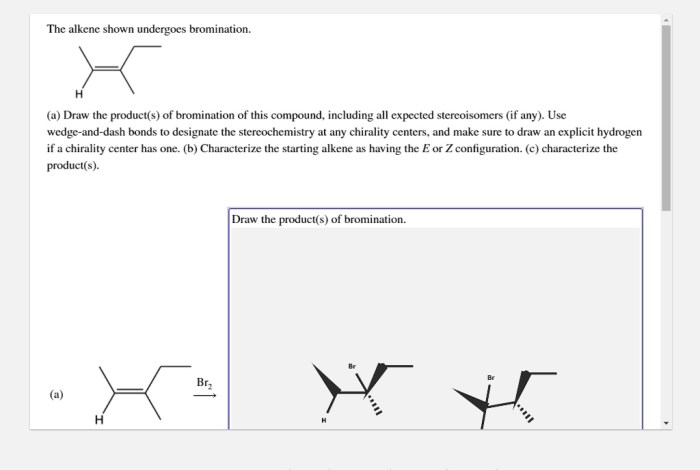
Alkene bromination is a chemical reaction in which an alkene reacts with bromine to form a vicinal dibromide. The reaction is initiated by the formation of a bromine radical, which then adds to the alkene to form a bromonium ion.
The bromonium ion is then attacked by a bromide ion to form the vicinal dibromide.
The mechanism of alkene bromination is as follows:
- Initiation: A bromine radical is formed by the homolytic cleavage of a bromine molecule.
- Propagation: The bromine radical adds to the alkene to form a bromonium ion.
- Termination: The bromonium ion is attacked by a bromide ion to form the vicinal dibromide.
An example of an alkene bromination reaction is the reaction of ethene with bromine to form 1,2-dibromoethane.
Regioselectivity in Alkene Bromination
The regioselectivity of alkene bromination is the preference for the reaction to occur at one carbon atom over another. The regioselectivity of alkene bromination is determined by the stability of the bromonium ion intermediate. The more stable the bromonium ion, the more likely the reaction is to occur at that carbon atom.
The following factors affect the regioselectivity of alkene bromination:
- The structure of the alkene: Alkenes with more substituted carbon atoms form more stable bromonium ions and are therefore more likely to undergo regiospecific bromination.
- The solvent: Polar solvents favor the formation of the more stable bromonium ion and therefore increase the regioselectivity of the reaction.
- The temperature: Higher temperatures favor the formation of the less stable bromonium ion and therefore decrease the regioselectivity of the reaction.
The regioselectivity of alkene bromination can be controlled by using a variety of methods, such as:
- Using a polar solvent
- Using a low temperature
- Using a bulky base
Stereoselectivity in Alkene Bromination, The alkene shown undergoes bromination
The stereoselectivity of alkene bromination is the preference for the reaction to occur with one stereochemistry over another. The stereoselectivity of alkene bromination is determined by the conformation of the alkene and the approach of the bromine molecule.
The following factors affect the stereoselectivity of alkene bromination:
- The conformation of the alkene: Alkenes with a more substituted carbon atom on the same side of the double bond are more likely to undergo syn addition.
- The approach of the bromine molecule: Bromine molecules can approach the alkene from either the top or the bottom. If the bromine molecule approaches from the top, the reaction is more likely to occur with syn addition. If the bromine molecule approaches from the bottom, the reaction is more likely to occur with anti addition.
The stereoselectivity of alkene bromination can be controlled by using a variety of methods, such as:
- Using a bulky base
- Using a polar solvent
- Using a low temperature
Applications of Alkene Bromination
Alkene bromination is a versatile reaction that can be used for a variety of purposes, such as:
- The synthesis of vicinal dibromides
- The synthesis of alkenes with specific regio- and stereochemistry
- The synthesis of other functionalized alkenes
Alkene bromination is an important reaction in organic chemistry and is used in the synthesis of a wide variety of compounds.
FAQ Section
What is the driving force behind alkene bromination?
The reaction is driven by the formation of a new carbon-bromine bond, which is energetically favorable due to the high electronegativity of bromine.
How can the regioselectivity of alkene bromination be controlled?
Regioselectivity can be controlled by factors such as the stability of the carbocation intermediate, the steric hindrance around the double bond, and the use of polar solvents.
What are the practical applications of alkene bromination?
Alkene bromination finds applications in the synthesis of pharmaceuticals, polymers, and other valuable compounds, including flame retardants, dyes, and fragrances.