In the realm of chemistry, solutions play a pivotal role, and among them, a solution of H2SO4 with a molal concentration stands out as a subject of significant importance. This article delves into the intricacies of H2SO4 solutions, exploring their properties, preparation methods, and diverse applications, providing a comprehensive understanding of their significance in various scientific and industrial domains.
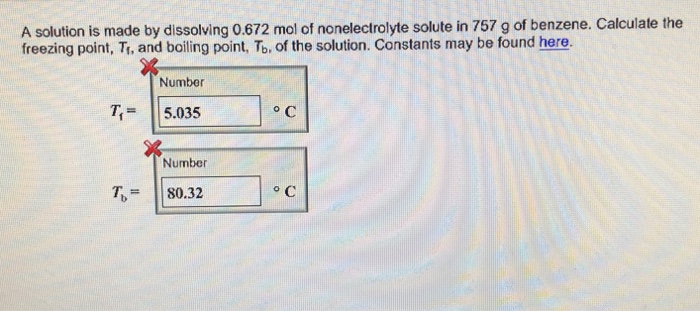
The concept of molality, a measure of concentration expressed in moles of solute per kilogram of solvent, forms the cornerstone of our discussion. By manipulating the molal concentration of H2SO4, we can tailor its properties and harness its potential for specific applications.
Introduction to Molal Concentration and H2SO4 Solutions

Molal concentration is a measure of the amount of solute dissolved in a solvent. It is defined as the number of moles of solute per kilogram of solvent. A solution is a homogeneous mixture of two or more substances. The solute is the substance that is dissolved in the solvent.
The solvent is the substance that does the dissolving. Solutions with different molal concentrations have different properties.
Properties of H2SO4 Solutions, A solution of h2so4 with a molal concentration
H2SO4 is a strong acid that is highly corrosive. It is used in a variety of industrial applications, including the production of fertilizers, dyes, and explosives. The molal concentration of an H2SO4 solution affects its physical and chemical properties. For example, the density of an H2SO4 solution increases with increasing molal concentration.
The viscosity of an H2SO4 solution also increases with increasing molal concentration.
Preparation of H2SO4 Solutions
H2SO4 solutions can be prepared by dissolving H2SO4 in water. The amount of H2SO4 that is dissolved determines the molal concentration of the solution. It is important to use the correct molal concentration for the intended application. For example, a solution with a high molal concentration of H2SO4 is used in the production of fertilizers.
A solution with a low molal concentration of H2SO4 is used in the production of dyes.
Applications of H2SO4 Solutions
H2SO4 solutions are used in a variety of industrial applications. Some of the most common applications include:
- Production of fertilizers
- Production of dyes
- Production of explosives
- Metalworking
- Oil refining
The molal concentration of an H2SO4 solution affects the effectiveness of these applications. For example, a solution with a high molal concentration of H2SO4 is used in the production of fertilizers. A solution with a low molal concentration of H2SO4 is used in the production of dyes.
Query Resolution: A Solution Of H2so4 With A Molal Concentration
What is the significance of molal concentration in H2SO4 solutions?
Molal concentration plays a crucial role in determining the properties of H2SO4 solutions, such as density, boiling point, and freezing point. By controlling the molal concentration, we can tailor these properties to suit specific applications.
How are H2SO4 solutions prepared?
H2SO4 solutions can be prepared by dissolving a known mass of H2SO4 in a known mass of water. The molal concentration is then calculated based on the moles of H2SO4 per kilogram of water.
What are some common applications of H2SO4 solutions?
H2SO4 solutions find widespread use in various industries, including chemical manufacturing, metal processing, and wastewater treatment. They are also employed in laboratory settings for acid-base reactions and as a drying agent.